(This is Part 6 of Dr. Pagtakhan’s column, Medisina at Politika , on Covid-19.)
Pandemic onslaught marches to the 2-million mark
In just 14 days since my last report, the world has seen the addition of over one million new patients with COVID-19. Compared to the 755,591-figure recorded two weeks earlier by the Johns Hopkins University, the world’s total stands at 1,883,119. Over half-a-million – 560,891– are in the USA. Canada’s share stands at 25,548 patients – a three-fold increase – more than half from Quebec (53%) and less than a third from Ontario (29%). British Columbia has 1,445 patients; Philippines has 4,428 patients.
Situation in Manitoba
Manitoba has reported 243 patients. “Premier Brian Pallister and the province's chief public health officer, Dr. Brent Roussin,” John Woods of Canadian Press reported last week, are “contemplating stricter measures and stepped up enforcement to ensure Manitobans follow social-distancing guidelines."
Today, CBC News headlines: “Manitoba extends public health orders under Public Health Act for 2 more weeks.” The orders “that shut down non-essential businesses and forbid public gatherings larger than 10 people” remain in force until April 28th.
Explanation for differences in clinical outcomes
News anchor George Stephanopoulos of Good Morning America said today: "I'm one of those cases that are basically asymptomatic. I've never had a fever, never had chills, never had a headache, never had a cough, never had shortness of breath. I'm feeling great." He was tested positive for COVID-19 as a close contact of his wife who said on her Instagram post on April 1st: “High fever. Horrific body aches. Heavy chest, never been sicker."
We know that coronavirus infection – the acquisition of SARSCoV-2 virus following exposure to a person carrying the virus – can result in different clinical outcomes among individuals in the same risk group: 1) respiratory failure and death; 2) severe pneumonia and recovery on oxygen therapy, with or without ventilator; 3) mild pneumonia and spontaneous recovery at home; and 4)asymptomatic carrier who may not even be aware and only detected when tested as a contact.
What we do not know is why the differences in response following exposure to the same virus. In an interview with the science magazine, Nature , Professor Arturo Casadevall at the Bloomberg School of Public Health and the School of Medicine at Johns Hopkins University and Professor Liise-anne Pirofski of the Albert Einstein Medical College have offered five scientific explanations to help us understand differences in clinical outcomes:
1) the amount of virus (the microbial dosage or number of viral particles);
2) our genes (which vary from person to person whether their body cells contain the ‘surface protein’ with which the virus gains access to the body);
3) the route of infection (whether the virus droplets inhaled through the nose trigger different immune defenses than the virus acquired by touching contaminated surfaces and then touching one’s face);
4) the strength of the virus variety (viruses differ in their capacity to damage the body and may undergo changes in their structure that enhance or weaken their capacity to do harm); and finally
5) the people’s immune status (“When a person’s immune system has no memory of an infectious agent, it may be unable to rapidly respond, and this may allow the invader to escape detection, giving it more time to cause damage.”)
Thus, it is impossible to predict who will end up as an asymptomatic carrier spreading the virus or who will succumb from the infection. This is the uncertainty and unpredictability COVID-19 poses to all of us and should help guide us as we receive directives from public health authorities.
The latest development on non-pharmaceutical treatments
Michael Doyle of the Globe and Mail recently reported: “Canada begins clinical trial of experimental COVID-19 treatment using plasma from recovered individuals.” The investigational treatment is based on the theory that “people who have recovered from COVID infection may have antibodies to fight the virus.” Perhaps the world’s largest clinical trial of this type of non-pharmaceutical treatment, the clinical trial “will involve injecting antibody-rich plasma from patients who have recovered from the virus into those who are still infected.” All patients who are hospitalized and need oxygen will be able to sign up for the trial. Investigators hope to enroll “1,000 patients from across the country and will include at least 40 Canadian hospitals.” The clinical trial will headed by Dr. Donald Arnold, a hematologist at McMaster University, and will be overseen by his team of doctors from his university and the University of Montreal, University of Ottawa, University of Toronto, and the University of British Columbia,” among other medical schools. “The clinical trial will have a control group – meaning that certain patients do not receive plasma. This is the gold standard for clinical trials and would be key in determining the widespread efficacy of this treatment.” Said Dr. Arnold: “It’s a flip of a coin – two-thirds of patients will get the antibody-rich blood, with the other third being a control group. The control group would receive the hospital standard care.” Dr. Michael Joyner of the Mayo Clinic will run the American national supply chain of plasma.
Portable and easy-to-use Canadian COVID-19 test kits now available
CBC News reported today that the Ottawa-based Spartan Bioscience Company has received Health Canada’s approval to ship its portable COVID-19 test kits to the federal government and its provincial partners. “The automated test can be operated by non-laboratory personnel in settings such as airports, border crossings, doctors’ offices, pharmacies, clinics, and remote communities,” says the Company. Until now, health officials have been doing targeted testing for COVID-19 because of a general shortage of testing materials and scarcity of trained staff. Now, there will be capacity to do widespread testing when required.
Federal call for volunteers
The federal government recently launched the National COVID-19 Volunteer Recruitment Campaign – inviting Canadians “who have the skill-set to do public health, like contact-tracing and other skills that provinces and territories are looking for,” to apply. The online process ends on April 24. The application form asks a series of questions to ascertain the applicants’ 1) knowledge and understanding of communicable disease prevention and control; 2) functional and content expertise in communications, public health, health care aide/nurse aide/ resident care work, laboratory work (viral, bacterial), quarantine, and 3) clinical experience as a physician. These are some of the skills needed and one does not need to have all. I certainly commend this opportunity to anyone with the skills set by volunteering. [I have volunteered.]

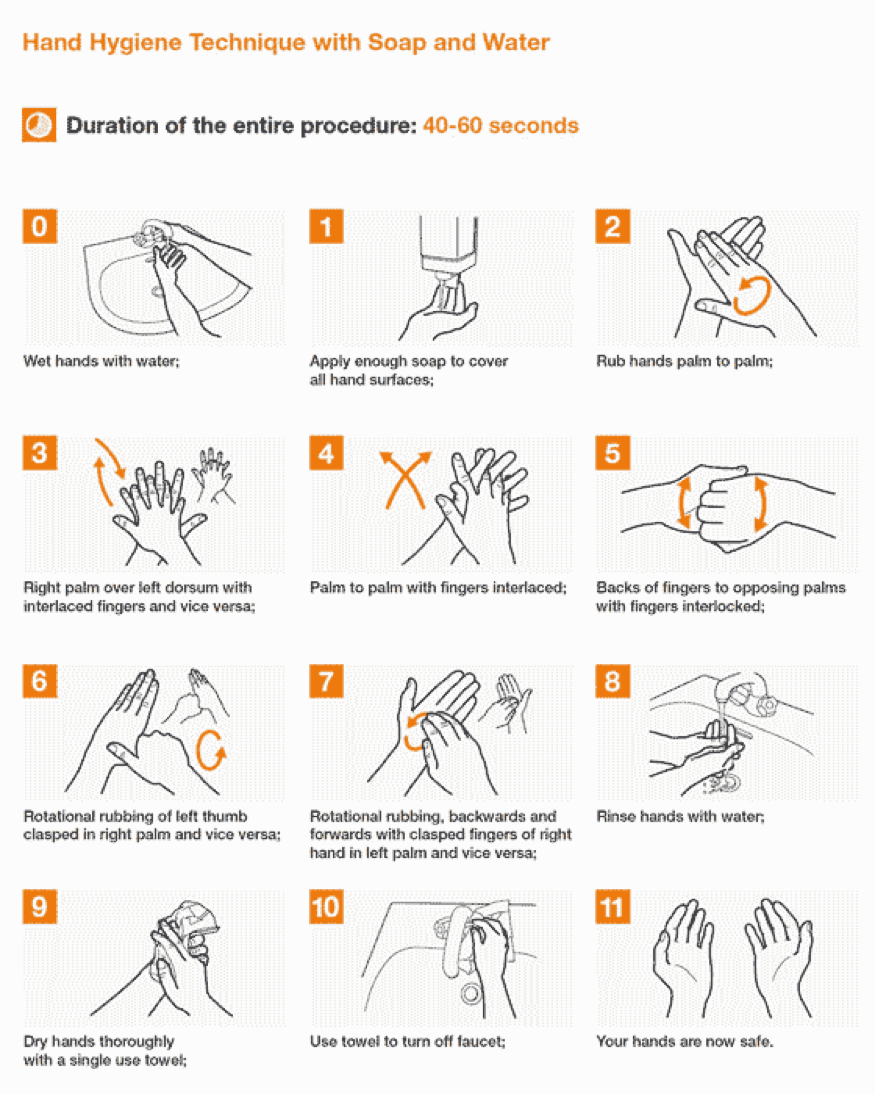
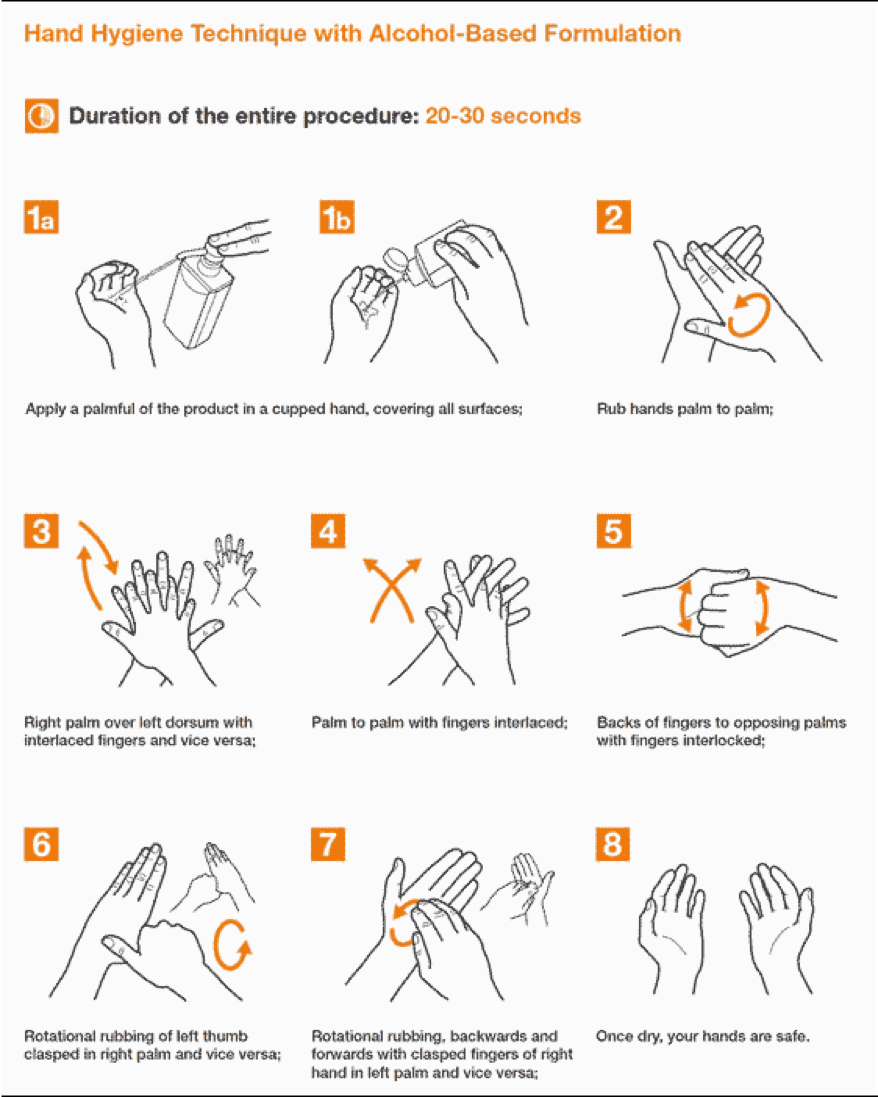
Hand-washing and Hand-rubbing Diagrams
Hand-washing with soap and water – the preferred procedure – and hand rubbing with an alcohol-based formulation are very important preventive measures against COVID-19. Proper techniques, including duration of the entire procedure, are important to ensure effectiveness. These diagrams come from the library of the World Health Organization.