(This is Part 11 of Dr. Pagtakhan’s column, Medisina at Politika, on Covid-19.)
Ground-breaking Development in Treatment – Saves Life
July 1, 2020 - Until two weeks ago before the Press Statement from the Chief Investigators of the Randomized Evaluation of COVID-19 Therapy (RECOVERY) Trial was issued on Tuesday June 16, medical science had no known drug to save the lives of critically COVID-19 patients requiring oxygen with or without the assistance of mechanical ventilator. Although remdesivir, a new drug, has been shown recently to shorten the time to recovery in hospitalized patients, it did not reduce mortality.
That Tuesday Dr. Peter Horby, one of the Chief Investigators for the trial and Professor of Emerging Infectious Diseases in the Nuffield Department of Medicine, University of Oxford, in England announced the preliminary results of their large-scale research study on an old commonly used anti-inflammatory drug: “Dexamethasone is the first drug to be shown to improve survival in COVID-19. This is an extremely welcome result. The survival benefit is
clear and large in those patients who are sick enough to require oxygen treatment, so dexamethasone should now become standard of care in these patients.”
“Dexamethasone is inexpensive, on the shelf, and can be used immediately to save lives worldwide,” the Professor further noted. He and his team issued the advanced statement in light of the global public health importance of the results. Added Associate Editor Molly Walker of MedPageToday: “Finally: Common Drug Improves COVID-19 Survival.”
The Randomized Evaluation of COVID-19 therapy (RECOVERY) trial is a randomized, controlled trial comparing a range of possible treatments with usual care in patients hospitalized with COVID-19. The Press Statement is on the preliminary results that compared dexamethasone 6 mg given once daily for up to ten days versus usual care alone. The primary outcome was 28-day mortality. 2,104 patients were randomly allocated to receive dexamethasone were compared with 4,321 patients concurrently allocated to usual care.
The results are impressive. Dexamethasone reduced deaths: (1) by one-third in patients receiving invasive mechanical ventilation; and (2) by one-fifth in patients receiving oxygen without invasive mechanical ventilation. Equally noteworthy is the finding that the drug did not reduce deaths in patients not requiring intensive or invasive respiratory support. They emphasized that they did not study patients outside the hospital setting and cautioned on the possibility of harm if used that way.
Professor Martin Landray of Medicine and Epidemiology at the Nuffield Department of Population Health, also at the University of Oxford and the other Chief Investigator, provided additional insights: ‘Since the appearance of COVID-19 six months ago, the search has been on for treatments that can improve survival, particularly in the sickest patients.” He continued: “COVID-19 is a global disease – it is fantastic that the first treatment demonstrated to reduce mortality is one that is instantly available and affordable worldwide.”
“To be clear, the benefit of using dexamethasone is seen in the sickest hospitalized patients,” said Dr.Bram Rochwerg, a critical care physician at McMaster University in Canada. “The drug should not be taken prophylactically or by stable outpatients with COVID-19.”
Observed Dr Jesus Villar, a critical care researcher at Dr. Negrín University Hospital in Spain: “And because the half-life of the drug is more than 36 hours, clinicians can give one intravenous dose a day, which simplifies treatment in the intensive care unit (ICU).”
“It’s a very safe, generally well-tolerated—at reasonable doses—steroid that works predominantly by its anti-inflammatory effects,” said Dr. Matthew Cheng, a medical microbiologist and infectious disease specialist at the McGill University Health Centre. “I was quite honestly elated. This is the first data that we have that suggests that a drug is able to improve the chances of survival in those that are critically ill, and so while I eagerly await the scientific publication I was absolutely thrilled to receive the news… we need to continue building upon them and potentially trying different combination strategies to further bring the mortality rates down. It should unequivocally be celebrated from the scientific community.”
On Tuesday in the UK, the chief medical officers of England, Wales, Scotland, and Northern Ireland informed all the doctors and hospitals that dexamethasone should become standard care for admitted patients with COVID-19 who require oxygen.”
Hand Sanitizers Alert – Danger and Recall
Health officials in New Mexico recently reported to CNN news (June 27th) that three persons died and four others became critically ill including one who became blind following ingestion of hand sanitizers containing methanol.

This incident alerts us (1) to existing reminder that these products, even when there are no impurities, pose a health hazard to toddlers; and (2) to Health Canada’s temporary modification on March 27 of its rules on hand sanitizer products that allowed manufacturers to use technical-grade ethanol versus food-grade ethanol to meet the pandemic demands for the products, then in short supply. The modified rules were for a short-term period and under specific conditions, including: 1) clearly indicating that technical-grade ethanol is included as an ingredient; 2) specific directions for use and warnings that these products are intended for adult use only; 3) that they should not be used on broken or damaged skin, or by women who are pregnant or breastfeeding, and 4) that they should not be inhaled.
Recently, Health Canada issued recall orders for the following sanitizers that use industrial-grade ethanol and contain the health-hazard contaminant ‘ethyl acetate’: Estraderm Hand Sanitizer 70% Ethyl Alcohol; Hand Sanitizer by Contract Packaging; Gel 700 Hand Sanitizer; Salinas Hand Sanitizer 70% Ethanol; Walker Emulsions Hand Sanitizer; Hand Sanitizer Disinfectant; Protectenol Hand Sanitizer Liquid; Tidol Hand Sanitizer 70%; Aktif Antiseptique instantane pour les mains; Smart Care Hand Sanitizer; X-Pure Vert-2-Go Gel; Gel Antiseptique
Pour Les Mains by Megalab; Germzero; Tekare Instant Hand Cleanser Gel; Dash Vapes Hand Sanitizer; and Isogel. Owners of these sanitizers are advised to promptly stop using them and to consult with their health care professional on any health concerns.
It is best to wash the hands with soap and water rather than use hand sanitizer, says Health Canada and thereby avoid the health hazards associated with impurities in sanitizers.
COVID-19 Virus Still with Us
(from Johns Hopkins University Tracking Dashboard - June 29)
In the half-year since its emergence and subsequent pandemic spread, COVID-19 has sickened more than 100-thousand Canadians, of whom over 8-thousand had died, while nearly two-thirds had recovered, with slightly over a quarter remaining active cases (see Table).
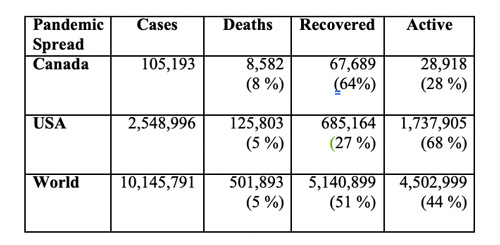
Neighboring USA has over 2.5 million cases, of whom over 125-thousands had died, with just over a quarter recovered, and a little over two-thirds still active cases. Globally, the pandemic has over 10 million people across 188 countries sick and half a million deceased. About 5 million have recovered, with slightly more than 4.5 million still active cases.
These are sobering numbers awaiting a preventive vaccine.