(This is Part 30 of Dr. Pagtakhan’s column Medisina at Politika on Covid-19.)
[Editor’s Note: In February 2020, CF Net started publishing Dr. Rey Pagtakhan’s column, Medisina at Politika, on COVID-19. Now on Part 30 of his running commentary, Dr. Pagtakhan’s twice monthly column has kept the Filipino community and other CF Net readers continually informed about 1) the pandemic and its impact, 2) the scientific advances on drugs and vaccines, and 3) the effectiveness of public health responses, including mass vaccination as a vital step to individual protection and development of community or herd immunity.]
‘COVID-19 vaccines are a triumph of science and a tool of medicine to prevent the disease; vaccination is what makes them save lives.’
Health Canada greenlights Pfizer-BioNTech’s COVID vaccine for younger adolescents 12 to 15 years of age – the first country to grant emergency use authorization for a pediatric vaccine. This is wonderful news and a milestone in the war against the pandemic.
Up until the 5th of this month, only the Pfizer-BioNTech COVID-19 vaccine of the four authorized for emergency adult use in Canada – the other three are Moderna, Astra-Zeneca and Janssen (Johnson & Johnson) – is also authorized for teens as young as 16 years old. On this date, Health Canada expanded the Pfizer authorization to include children 12 to 15 years of age. The efficacy is 100%; it is well-accepted and safe; and the vaccine-maker is firmly committed, post-authorization, as part of the vaccine’s approval “to monitor the long-term safety, efficacy and quality of the vaccine and to provide the information to Canada’s health ministry.” Five days later, the U.S. also granted expanded authorization to the Pfizer-BioNTech vaccine.
Reasons why vaccinating children against COVID-19 is crucial
Even before any COVID-19 vaccine was granted emergency use authorization for adults in Canada, the U.S. and Europe, the Director of Child Health at the International Vaccine Access Center of the Johns Hopkins Bloomberg School of Public Health Dr. Anita Shet had advanced in mid-December 2020 “five broad reasons why children should get a COVID-19 vaccine”:
- It will facilitate the development of herd immunity and, thereby, in the control of the pandemic since children constitute a large proportion of the population (not quite a fifth in Canada, close to a quarter in the U.S., and a little over a third globally);
- It can reduce the overall burden of the infection at home and in the community since older children and adolescents transmit the COVID virus to their contacts– to parents, grandparents, and other adults;
- It will decrease the total burden of disease on the health care system since children, although to a lesser extent than adults, can become seriously ill and die;
- It will save children from the devastating indirect effects of COVID-19 on their education and mental health since an increased number of them showed failing grades during the era of remote learning and frequent emergency department visits for mental health issues; and
- It will ensure children would be treated fairly as adults, that is, receive the benefits of vaccines as well as contribute to their clinical trials, as advocated in writing by the American Academy of Pediatrics to federal health officials, “The observation that children appear to have lower risk of severe COVID-19 disease compared with adults should not result in their exclusion from vaccine clinical research.”
Dr. Shet went on to emphasize the half-dozen well-known observations in pediatric clinical research support of the above-noted broad rationale:
- “Children are not ‘little adults’ – their physical, metabolic, immunological, and psychological processes are different from those of adults, (and these) often translate to children having stronger reactions such as higher fever and localized reactions; (hence,) separate safety data in children must be meticulously collected and critically studied before recommendations can be made for deployment in children;
- “efficacy data are important to compile in different age categories (from infancy through childhood) given the differential immune responses in different ages…to identify optimal (age-specific) pediatric doses;
- “long-term monitoring of vaccine safety within trials and in the general population is critical to building a strong safety profile of the vaccines that are to be used in children;
- “running pediatric vaccine trials is more complicated compared to adult trials and an integrated and sustainable trials network geared towards pediatric approaches is necessary…to provide the infrastructure and rigor needed to improve the safety and efficacy of (vaccines) for children;
- “an open channel of communication with the public with complete transparency of what is known and unknown is…needed to build vaccine confidence; and
- “(clinical) trial enrollments should reflect the racial and ethnic diversity of the (local) and global populations…and (thereby) contribute to reducing inequities among societies.”
In support of Dr. Shet’s position, Dr. Anna Durbin (Professor) and her colleagues Drs. Ruth Karron (Respiratory Virologist and Vaccinologist), William Moss (Executive Director), and Kawsar Talaat (Assistant Professor)in the Department of International Health and the Center for Immunization Research, also at the Johns Hopkins Bloomberg School of Public Health, have more recently (February 25, 2021)indicated that “more than 10,000 children have been hospitalized with Covid-19 in the U.S. and more than three hundred children have died.” They specifically noted a serious complication of the disease in children, called Multisystem Inflammatory Syndrome in Children (MIS-C) which “can itself lead to death or long-term complications.”
With more adults vaccinated, children now constitute about 24% of new COVID-19 cases according to the American Academy of Pediatrics. In Canada, approximately 20% of the more than 1.2 million COVID-19 cases are in the pediatric population.
The clinical trials for the pediatric vaccine
Evaluation of the Pfizer-BioNTech vaccine in children with respect to its safety and ability to induce the required immune response started in the U.S. sometime in October 2020. Enrollment in the clinical trial included 2,259 pediatric participants 12 to 15 years of age, half of whom received the vaccine and half a placebo, in a double-blind design.
Note that the number of participants is much smaller than in the trials in adults. This is because the trial in teens was the so-called “immune bridging” technique, which is designed to test whether the vaccine triggered immune responses similar to those in adults, that is, “to expand access to vaccines that have been proved effective and safe to older populations.”
Clinical trials were done at university medical centers under the close supervision of experienced pediatricians and their respective clinical research teams. Dr. Robert Frenck, the Director of the Gamble Center for Clinical Research at Cincinnati Children's Hospital Medical Center in Ohio, observed that “children actually appear to respond better to the vaccines than younger adults. The challenge now is to find the best immune response with the lowest side effects."
The results formed the basis for Pfizer’s application for expanded authorization. It had already included children as young as 16 years of age in its original application for emergency use. In addition to achieving the two primary aims of the trial among younger subjects – (1) to ascertain safety and (2) to assess the immune response – the results also show that more children became ill in the placebo group thereby confirming the strong protection as in adults the vaccine offers.
The Moderna pediatric vaccine trial, which has enrolled up to 3,000 children and adolescents 12 to 17 years of age – also in the U.S. – expects its results and favorable application for expanded authorization sometime this summer. Its original authorization was only for adults 18 years and older. Clinical trials in children younger than 12 years of age and as young as six months old are either planned or already underway, but completion is not expected earlier than the following year.
AstraZeneca, Johnson and Johnson, and other vaccine-makers have not yet applied in North America for clinical evaluation of their vaccines in the pediatric population.
Importance of clinical trials in building vaccine trust and confidence
It cannot be gainsaid that building confidence in the trustworthiness of Covid-19 vaccines for children is critical. It would go a long way in overcoming vaccine hesitancy and convincing more parents to bring their infants and children for immunization. In recognition of this need and with the support of the Public Health Agency of Canada, the Canadian Pediatric Society has developed for its members a learning module to “counter misinformation about COVID-19 with clear, evidence-based information; initiate discussions regarding specific vaccine issues; recognize personal skepticism regarding the safety and efficacy of COVID-19 vaccines; respectfully address vaccine hesitancy, using clear, evidence-based information; and build public confidence regarding the safety and efficacy of COVID-19 vaccines.”
Canada’s reception of the pediatric vaccine
News writer Alexandra Mae Jones (CTVNews. May 5, 2021) surveyed the provinces and territories on their reactions to the expanded authorization of the Pfizer vaccine to include adolescents 12 to 15 years of age. All welcomed the news with most indicating the doses will be available to all youth immediately as supplies allow (Alberta, NWT); certainly, by mid-June or before the end of the school year (B.C., Saskatchewan Manitoba, Quebec, New Brunswick); completed late September and very early in the next school year (PEI and Newfoundland and Labrador); and others with no definitive timeline yet (Ontario). ink that
Insights from clinical trial participants – children and parents
I thought I should share some inspiring and true-to-life feelings from some of the adolescent participants and their parents as gleaned from the remarks they had shared with the media. They tell a story.
From the children, I read of these as reported by the media:
- “I just trusted the science; I knew it was tested in adults. I was really just joining, hoping that maybe I could get vaccinated and help out science.”
- "I wanna hang out with friends, go to movies, take my mask off as soon as possible."
- “I think that it could really benefit the world, and I think it could also help scientists know more about the coronavirus.”
- “It’s really not scary. There’s nothing dangerous or intimidating about it; I hope others my age join the study.”
- “They were talking about symptoms… fatigue, low-grade fever, headache. I hope I don’t have anything like that because I don’t want it to mess with school or work, but I wasn’t thinking about my permanent health for a short-term inconvenience.”
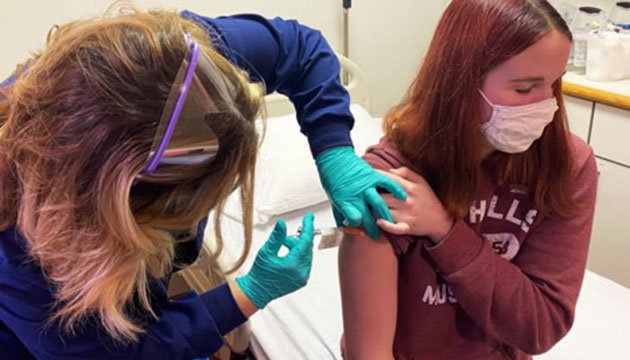 Katelyn Evans, 16, receives a Covid-19 vaccine during a trial at Cincinnati Children's Hospital Medical Center on October 14, 2020. [Credit: Elizabeth Chuck: NBC News. October 28, 2020. Photo Courtesy from the Cincinnati Children’s Hospital Medical Center]
Katelyn Evans, 16, receives a Covid-19 vaccine during a trial at Cincinnati Children's Hospital Medical Center on October 14, 2020. [Credit: Elizabeth Chuck: NBC News. October 28, 2020. Photo Courtesy from the Cincinnati Children’s Hospital Medical Center]
 Audrey Baker, 15, and Sam Baker, 12, participated in the Pfizer-BioNTech coronavirus vaccine trial at Cincinnati Children's Hospital Medical Center. They do not know yet whether they received the vaccine or a placebo. [Credit: Carolyn Y. Johnson: The Washington Post. May 10, 2021. Photo Courtesy by the Baker Family.
Audrey Baker, 15, and Sam Baker, 12, participated in the Pfizer-BioNTech coronavirus vaccine trial at Cincinnati Children's Hospital Medical Center. They do not know yet whether they received the vaccine or a placebo. [Credit: Carolyn Y. Johnson: The Washington Post. May 10, 2021. Photo Courtesy by the Baker Family.
From the parents, these they said to the media:
- “I’m happy that he’s doing his bit for science.”
- “With the Pfizer study, no major side effects have been reported so far, so that made me comfortable with enrolling him as well. By being part of this study, we help others. It’s a small contribution to the field.”
- “I imagine many parents these days would think I was quite nuts, but I think the thing about a clinical trial is there are lots of safety mechanisms built into that, so you’re not totally in the dark, and it’s really exciting to be involved in research.
- “She’s got a big heart. She obviously had no fear of this. I guess I’m more afraid of Covid than I am of the vaccine. It was excitement, it was pride, it was an understanding that life is about more than just you.”
These are touching statements.
 Heather Hannon with her daughter, Annelise, and son, Linus, seen in 2009 about a month before the kids participated in a vaccine trial. Now 16 and 14, the adolescents participated again in the Pfizer COVID-19 vaccine trial. [Credit: Elizabeth Chuck: NBC News. October 28, 2020. Photo courtesy of the mother, Heather Hannon, an oncology nurse practitioner]
Heather Hannon with her daughter, Annelise, and son, Linus, seen in 2009 about a month before the kids participated in a vaccine trial. Now 16 and 14, the adolescents participated again in the Pfizer COVID-19 vaccine trial. [Credit: Elizabeth Chuck: NBC News. October 28, 2020. Photo courtesy of the mother, Heather Hannon, an oncology nurse practitioner]
Words from world renown pandemic expert
Indeed, a pediatric vaccine is a milestone in the war against the pandemic. It will help protect the children themselves, their peers with underlying medical vulnerabilities which place them at increased risk, and the community at large as part of the population pool for herd immunity. Not only it would help us put the pandemic behind us but also it would help reduce the chances of future outbreaks.
On this note, let me conclude with the words of Dr. Anthony Fauci, world renown expert on pandemics and Chief Medical Adviser to President Joe Biden: "Now that we can vaccinate those kids, it's gonna make it much, much easier to get those kids back to school without the anxiety associated with whether or not there's gonna be an outbreak at that level."


![A 12-year-old boy participates in Pfizer's COVID-19 vaccine trial at Cincinnati Children's Hospital Medical Center last year. [Credit: Rebecca Renner: Science Coverage, National Geographic. February 1, 2021. Photograph Courtesy of the Cincinnati Children’s Hospital Medical Center.]](/images/photos/may2021/12-year-old.jpg)











