(This is Part 18 of Dr. Pagtakhan's column, Medisina at Politika, on Covid-19.)
The global breaking news on the night of November 3rd would be on the incoming U.S. President: President Trump seeking re-election or challenger former Vice-President Biden.
The global interest is palpable since we live in an interconnected world and the outcome would project to the world how the U.S. would, henceforth, handle the COVID-19 pandemic and its consequential impact on the economy, these being their two key election issues. Would we see a change in direction or would it be much of the same as before? At stake are human lives and livelihoods.
The Guardian – an independent journalism group owned neither by stakeholders nor by a billionaire – wrote in its October 27theditorial analysis, It’s time to dump Trump. America’s only hope is Joe Biden: “Mr. Trump seems incapable of acknowledging the suffering of others. Coronavirus has exposed a devastating lack of presidential empathy for those who have died and the families they left behind.”

The Table below shows the bi-monthly update on the COVID-19 human toll for the world, Canada, Philippines and the U.S. as per the Johns Hopkins University coronavirus tracker:
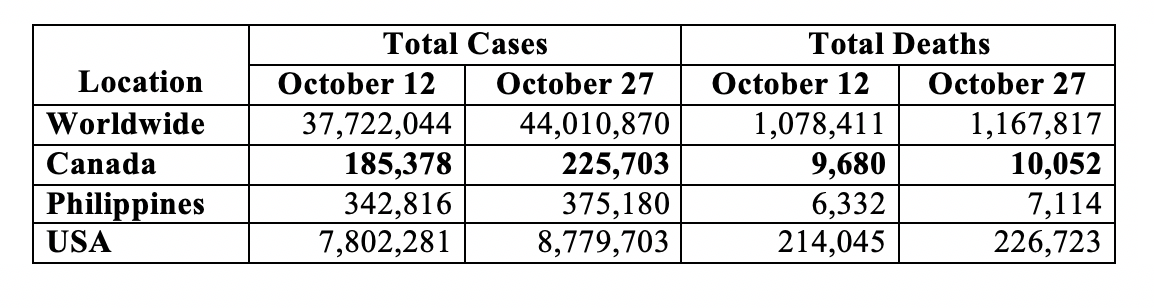
The world now has over 44 million cases and over 1.16 million deaths. Canada has over a quarter of a million cases and over 10,000 deaths. The USA has remained the epicentre – over 8.7 million cases and over a quarter of a million deaths – a daily average of just over 65,000 cases and 845 deaths in the half-month period since my preceding update. For Canada during the preceding half month, the average daily toll comes to just under 2,700 cases and 25 deaths. These numbers reflect recent surges not only in Canada and the U.S. but also in Europe – France, Germany, Italy and U.K. among others.
- Drugs and Vaccines
There are now two medications. Both Health Canada and the U.S. Food and Drug Administration have authorized the use of remdesivir (brand name ‘Veklury’) for adult and pediatric (12 years and older weighing at least 40 kg or 88 lbs) patients hospitalized with severe pneumonia and requiring oxygen therapy. The drug – the first new anti-viral medication authorized for use – should only be administered in a hospital or in a healthcare setting capable of providing acute care comparable to inpatient hospital care. Clinical trials assessing the safety and efficacy of Veklury in the pediatric patient population younger than 12 years old are ongoing.
The second drug is dexamethasone- already an existing drug in use for other clinical conditions. While it has now been recommended for hospitalized COVID-19 patients on mechanical ventilators or supplemental oxygen, note has been made that the drug may be harmful if given for less severe COVID-19 infection.

In contrast to medications, vaccines are envisioned not to cure but to prevent infection and disease. J. Corum, S. Wee and C. Zimmer updated on October 29 the readers of The New York Times on the categories of vaccines under development and investigation and on the testing process and clinical trials. The four categories of vaccines under study include the following:
i. Genetic Vaccines –they deliver one or more of the coronavirus’s own genes into our cells to provoke an immune response;
ii. Viral Vector Vaccines –they contain viruses engineered to carry coronavirus genes. Some viral vector vaccines enter cells and cause them to make viral proteins. Other viral vectors slowly replicate, carrying coronavirus proteins on their surface;
iii. Protein-Based Vaccines –they contain coronavirus proteins but no genetic material. Some vaccines contain whole proteins, and some contain fragments of them. Some pack many of these molecules on nanoparticles; and
iv. Inactivated or Attenuated Coronavirus Vaccines – they are created from weakened coronaviruses or coronaviruses that have been killed with chemicals.
The vaccine testing process follows a series of steps from the laboratory to the clinic. Vaccines are tested for efficacy and safety. Some will fail in the human trials; some may end without clear results; and some, hopefully, will succeed. Then comes the manufacturing on the scale, storage and deployment. While it took many years for existing vaccines for other diseases to be available, the current search for vaccines to COVID -19 has been hastened, thanks to the early identification and characterization of the virus and to the sharing of the information among research scientists worldwide.
Phase 3 clinical trials involve thousands of people receiving the vaccine and thousands more receiving a placebo. Comparison of results would determine whether the candidate vaccine protects against COVID-19. In the U.S., vaccine makers have been advised that the regulatory body for approval – the Food and Drug Administration (FDA) – would want to see evidence of at least 50% efficacy, that is, the vaccine can protect at least 50% of those who receive it.
Moreover, the very large size of volunteers participating in the clinical trials would provide additional assurance of safety since any potential relatively rare side effects that might have been missed in earlier studies would be known. That is why vaccines from countries that have approved them without requiring sufficient Phase 3 clinical trials pose serious risks.
The first human safety trial began in March this year. On October 25, Nada Bashir of CNN News reported on when potential vaccines might be available, quoting Dr. Anthony Fauci of the U.S. National Institute of Health: "We will know whether a vaccine is safe and effective by the end of November, beginning of December. The number of doses that will be available in December will not certainly be enough to vaccinate everybody -- you'll have to wait several months into 2021."
Three days later on October 28, Sharon Kirkey of the National Post reported on the collective assessment by 28 experts polled by Jonathan Kimmelman, a professor and director of the Biomedical Ethics Unit at McGill University. His team surmised that “a COVID-19 vaccine is most likely to be available to the general public in the United States and/or Canada (in) June 2021 for the soonest, but more likely fall of 2021.”
Non-pharmaceutical public health measures
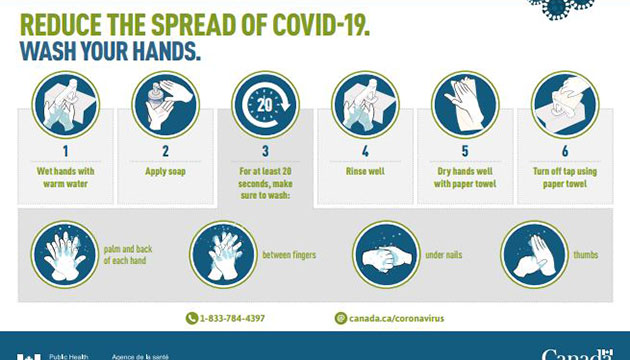
Even without vaccine, our personal behavior matters in the spread of the virus. Fortunately, we have proven non-pharmaceutical public health measures – both personal and community – that can prevent individual infection and community transmission. We cannot over remind ourselves about the necessity to keep doing the five safety measures and avoiding the three Cs.
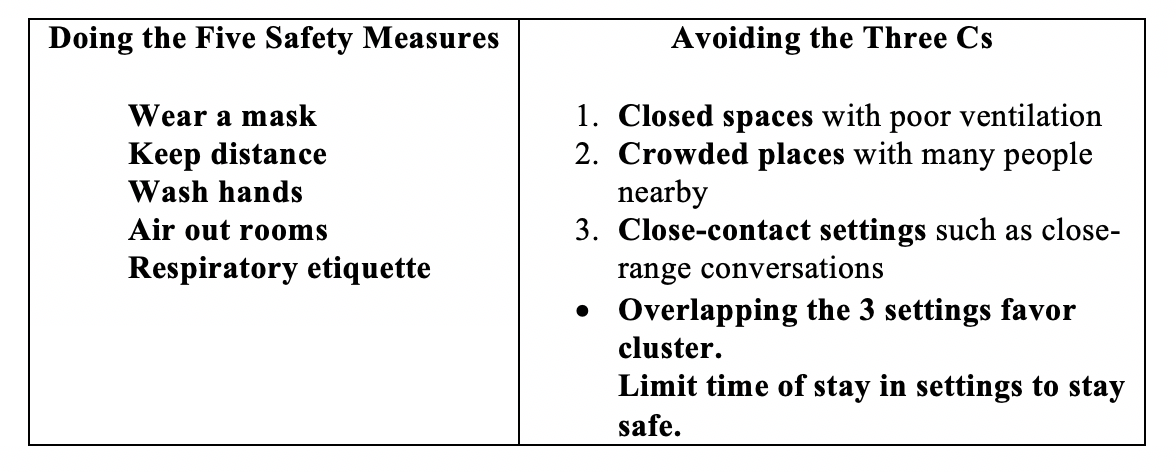
Said Dr. Anthony Fauci, America’s top COVID expert on public health measures, in a recent interview hosted by the University of Melbourne and the Melbourne-based Doherty Institute and reported on October 29 by Naaman Zhou in the Guardian Australia: “I firmly believe that you can continue to open businesses, …to open up the country from an economic standpoint … but you could do it prudently … by public health measures that prevent surges of infection.”
Handling of the Pandemic
A week ago, on October 25, U.S. President’s Chief of Staff Mark Meadows told Jake Tapper on CNN’s ‘State of the Union’: “We are not going to control the pandemic.”
This is what ‘natural herd immunity’ as a policy strategy is: “the government should let the pandemic run its course until most of the population (70%) is infected and has ostensibly developed antibodies to ward off future infections.” Except, it is against the consensus of public health experts.“Try to reach it without a vaccine, and millions will die,” said Carl T. Bergstrom (Professor of Biology, University of Washington) and Natalie Dean (Assistant Professor of biostatistics, University of Florida) to The New York Times.
Added Dr. Ashish Jha, Dean of the Brown University School of Public Health in Providence, Rhode Island: "… no serious public health person actually thinks herd immunity is a good policy strategy." Jeffrey D. Sachs, a professor and director of the Center for Sustainable Development at Columbia University remarked to CNN interview: “As if (the U.S. President’s) irresponsibility was not already a national tragedy, the White House seems now to favor a controversial approach to Covid-19 that threatens to bring nothing less than mass suffering.”
Canadian columnist Andrew Coyne succinctly summed his observations on the subject for the October 16 issue of The Globe and Mail: “Herd immunity’s a great strategy if you don’t mind the millions of dead.”
Avoidable Deaths and their Wide Circle of Tragedy
Evidently, the arrival of the two medications (the new remdesivir and the old repurposed dexamethasone) for a particular phase of hospitalized COVID-19 patients – those with severe pneumonia requiring oxygen only and those additionally requiring mechanical ventilator, respectively – has prevented a number of deaths that would have otherwise occurred. Non-pursuit of disease-induced herd immunity is one other big factor that would prevent unnecessary deaths. Likewise, compliance with public health measures – non-pharmaceutical tools without any adverse side effects of a drug – has also prevented deaths by preventing infections to begin with. The latter, combined with the implementation of timely and appropriate health policies, guidance, coordination and political leadership, as discussed below, provides a fourth force for avoiding or reducing fatalities.
Recently, on October 21, Dr. Irwin Redlerner and his team at the National Center for Disaster Preparedness of Columbia University in New York released its report entitled “130,000 – 210,000 Avoidable COVID-19 Deaths – and Counting – in the U.S.” His team compared the U.S. fatalities (as of October 16) per 100,000 population to those of six other high-income nations, including Canada, which undertook “appropriate public health policies, guidance, and leadership.” The University team estimated “that at least 130,000 deaths and perhaps as many as 210,000 could have been avoided with “earlier policy interventions and more robust federal coordination and leadership.” Their report concludes: “The U.S. should have – and could have – done better to protect the nation, and particularly its most vulnerable populations….has turned a global crisis into a devastating tragedy.”
How ironic it is since “only last year the U.S. ranked first in the world in an independent assessment of pandemic preparedness” as rated by The Global Health Security Index. [The Index – a project of the Nuclear Threat Initiative and the Johns Hopkins Center for Health Security and was developed with The Economist Intelligence Unit – aims to improve the international capability to address one of the world’s most omnipresent risks: infectious disease outbreaks that can lead to international epidemics and pandemics.]
The Wide Circle of Tragedy
The Columbia University report also explored the “wide circle of tragedy with every COVID-19 death…. that no death is ever just ‘a number’ or simply part of a large database for disease tracking purposes.” It noted that the deceased – parents, grandparents, friends and children – have left “behind people who grieve and families that must struggle to regain economic stability.” Below is a partial list of consequences:
- Children
a. pushed into or near poverty as a consequence of the overall economic impacts of the pandemic;
b. risk of foster care placement as a result of losing their single parents to the disease; - Bereavement
a. well over 1.5 million Americans are grieving the approximately 167,000 avoidable deaths of a loved one (based on a “bereavement multiplier of 9 for every death” as developed by researchers at the Penn State University); - Long-term impacts and disabilities
a. long-term damage to patients’ lungs, heart, immune system, and brain;
(1) 78 out of 100 recovered COVID-19 patients had “abnormal findings on cardiovascular imaging (MRI)”
(2) 36 of 100 had difficulty breathing and unusual fatigue months after their diagnosis.
The above-mentioned observations call for a diligent follow-up by government(s) on the pandemic’s impact on Canadian families and long-term health.