(This is Part 22 of Dr. Pagtakhan's column, Medisina at Politika, on Covid 19.)
Joy to the world! How exciting it is, indeed, to know that Canadians, Americans, Britons and citizens of the European Union (EU) and 15 other countries have begun their mass immunization against COVID-19 to curb the unrelenting increase of new cases and deaths from SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2). The successful launch of this immunization drive before 2020 ended makes realistic the prospects of ending the acute pandemic before the end of the new year. Thanks to the regulatory agencies, timely issuance of emergency use authorizations (EUA) has allowed prompt arrival of both the Pfizer-BioNTech’s and Moderna’s COVID-19 vaccines. Already members of the targeted most vulnerable seniors and frontline workers have received the first of the two-dose intramuscular jabs to end the COVID-19 pandemic.
‘Shots of Hope’
‘Shots of Hope’wrote science-writer Jon Cohenin an essay on the two vaccines for the magazine, Science. He chronicled the evolution of the pandemic from its start in December 2019 in China before its causative virus was identified and the new disease fully characterized, and before their scientific and medical names were formalized, respectively. He wrote of his daily phone conversations with his 90-year-old mother who went through a period of denial before agreeing to shelter-in, repeatedly asking “When is this going to end?”
With that as background, Cohen then traced the search for vaccines after the pathogenic virus was identified on January 8, 2020 and its genetic sequence posted online by the Chinese scientists two days later. Apparently, the search for vaccines started within hours of January 10. He noted the “confluence of forces” that propelled science to two COVID-19 vaccines at breakneck speed with several more nearing completion of their Phase III clinical trials. He noted that “never before have governments, industry, academia, and non-profits thrown so much money, muscle, and brains at the same infectious disease in such short order.”
Overwhelming Yet Cautious Optimism
Indeed, what a joyous feeling to end 2020 and begin 2021 with overwhelming optimism. I never imagined the vaccines would be realized this soon, although I have prayed like others since I wrote the first part of this series for the February 1, 2020 issue to alert and inform our community and readership. Public health is now best served with two, not one, vaccines – a twin set of biomedical tools with 95%-efficacy and without concern of serious harm. Truly a scientific breakthrough!
Although we temper our optimism, I trust there would be no breakdown in the manufacturing supply chain. We remain confident any unexpected serious adverse event would be fully investigated with dispatch. In fact, the U.S. regulatory agency convened last week a panel of experts to analyze and discuss the reported case of severe allergic reaction in one vaccine.
The Current Situation
The launch of mass vaccination is timely. Globally, the half-month increase in the total diagnosed came to over 9 million cases, that is, an average of over half a million cases daily and the total cumulative mark has surpassed the 80-million mark. Deaths increased by Figue10% - a total daily average of over 10,000 (see Table below). Compared to the global figures, Canada showed a half-month increase of over 90,000 cases, that is, a total daily average of over 6,000. Deaths increased by almost 12% - a daily total average of over 100. For the U.S.A., the half-month increases expressed in per cent in both cases diagnosed and deaths seen were similar in magnitude to that of Canada. The increase in cases and deaths for the Philippines over the same half-month period was considerably less in magnitude. We need to stop the continuing circulation of the virus.
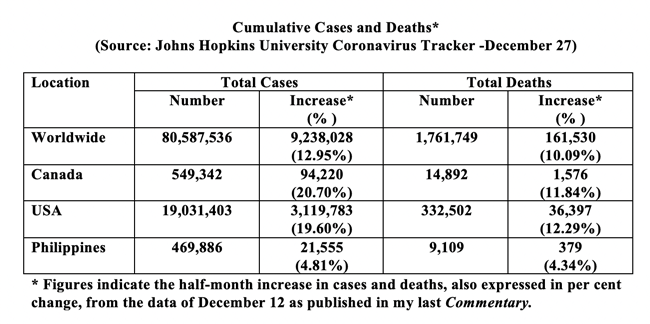
Overall, the figures remain alarming. Compliance with public health restrictions has become increasingly difficult. The feeling of helplessness among all concerned – families, communities-at-large, frontline health care workers, students, public health officials, businesses, and governments at all levels - appears clearly palpable.
What a change in feelings, indeed, when we heard about the vaccines being given the green-light for emergency mass vaccination. As discussed in the last issue. I chose to comment on the first one – the Pfizer-BioNTech COVID-19 vaccine – in more detail to emphasize the meticulous care applied to its rigorous evaluation and the strict criteria used by the regulatory agencies in Canada, Britain and the USA. To enhance public confidence in vaccines by public transparency, the U.S. Center for Biologics Evaluation and Research has published master protocols for SARS-CoV-2 (the COVID-19 virus) vaccine safety and effectiveness evaluation. Speed in approval was achieved without any short cuts. The regulatory agencies through their panel of experts have seen to it that safety and efficacy are paramount.
Comparison of Vaccines
Two weeks before Christmas, the U.K. government announced that the developers of the Oxford-AstraZeneca vaccine had submitted their application for authorization to the Medicines and Healthcare products Regulatory Agency (MHRA). Approval is expected to be granted on Jan. 4, 2021. With comparable levels of efficacy and safety and with having the advantage of lower cost and less onerous demands on transport and storage, the third vaccine would certainly boost the earlier two. More than 700 million doses are expected by the end of the first quarter of the new year.
Meanwhile, let us compare Pfizer’s and Moderna’s vaccines. Like Pfizer's, Moderna's vaccine delivers messenger RNA, or mRNA which is a” genetic recipe for making a piece of the spikes that sit atop the coronavirus.” Once injected, the body's immune system makes antibodies to the spikes. If a vaccinated person is later exposed to the COVID-19 virus, the person’s immune system is fully prepared to attack the virus.
We do not know how long protection will last following vaccination but it will be critically important to measure long-term protection (at least two years) in the phase 3 trials and in other groups prioritized for early vaccination. We are still learning about the duration of protection following infection with SARS-CoV-2 and it is too early to tell how long protection will last.
The two vaccines are of the same type, have similar efficacy levels, but do have slightly different makeup in their lipid delivery systems. That difference explains why they have different storage and handling features. Unlike Pfizer’s vaccine, Moderna’s vaccine does not have to be kept at super-cold environment. It can be kept at about minus-20 degrees Celsius, or about the temperature of a home freezer. Moderna's vaccine can also be kept in a refrigerator for 30 days before it expires. That makes it more manageable in terms of distribution to outlets.
Moderna's vaccine is administered as two 100-microgram doses given 28 days apart. Pfizer's vaccine is administered as two 30-microgram doses given 21 days apart to as young as 16 and older; for Moderna, the lower age range is at 18. The two share the same spectrum of transient side effects.
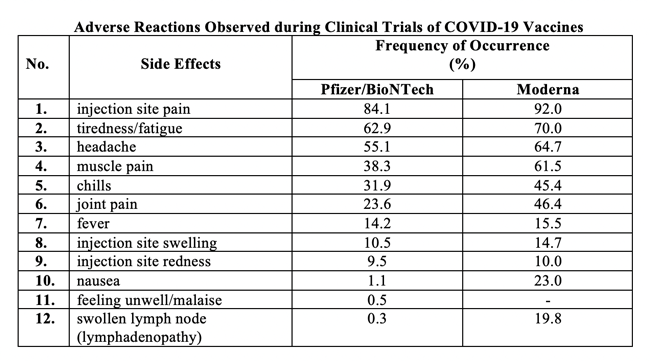
Side Effects Observed with the Two Authorized Vaccines
The table below summarizes the side effects and the frequency of their occurrence with the two authorized vaccines in Canada. Although I have listed them in one Table, I should note as remarked in the Fact Sheets for the vaccines, that “because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of one vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.” I have done it this way to save space
Additional Notes on Safety, Precautions and Contraindications
- There is a remote chance that either of the Pfizer’s or Moderna’s COVID-19 vaccine could cause a severe allergic reaction which, if it were to occur, usually occurs within a few minutes to one hour after getting a dose of the vaccine.
- Such reactions could include a bad rash all over the body, dizziness, weakness, fast heartbeat, swelling of face and throat, and difficulty breathing – these need emergency attention.
- Health Canada has, in fact, recommended that anyone who is allergic to any of the ingredients should not receive the vaccine. It also advised that anyone who has had a serious allergic reaction to another vaccine, drug or food to talk to their health professional beforehand.
- There are no data available on the interchangeability of the Pfizer‑BioNTech COVID‑19 Vaccine or the Moderna with other COVID‑19 vaccines to complete the vaccination series. Individuals who have received the first dose of the vaccine should receive a second dose of the same brand to complete the vaccination series
Scarcity and Allocation of Vaccines
Even with the Oxford/Zeneca and other additional candidate vaccines in the pipeline, there won’t be enough for the general population until spring, and doses will be allocated to groups based on degree of vulnerability to COVID-19. Apparently, Canada is on track to receive 1.2 million doses of both vaccines by the end of last month. The vaccination campaign for the general population will begin in April. These are Canada's vaccine orders to date, including options:
- Pfizer — Up to 76 million doses (minimum of 20 million doses for delivery this year 2021.)
- Moderna — Up to 56 million (Canada has so far agreed to purchase 40 million doses.)
- Medicago — Up to 76 million
- AstraZeneca — Up to 20 million
- Johnson & Johnson — Up to 38 million
- Novavax — Up to 76 million
- Sanofi and GlaxoSmithKline — Up to 72 million
- International COVAX Facility — Up to 15 million
Maj. Gen. Dany Fortin, the commander in charge of Canada's vaccine logistics, said the distribution last month of the Pfizer vaccine had gone smoothly. The provinces and territories have largely adhered to the guidelines released by the National Advisory Committee on Immunization. Long-term care and retirement home residents and staff, the elderly, front line health care workers and some Indigenous adults are in the first phase of distribution.
Considering the global supply, it is understandable to have scarcity. Obviously, continued expansion of vaccine manufacturing capacity and availability of other vaccines are needed to meet demands, including global access. Meanwhile, we shall continue to include patience as a principle in addition to the principle of equity.
Diversity and Inclusion of Minorities in Vaccine Trials
It is gratifying to note that, from the viewpoints of both public health science and social justice, participants in the clinical trials of the two COVID-19 vaccines that have received emergency use authorizations in Canada, the United States, the United Kingdom, and the 27-nation bloc of the European Union have included representations from minorities. They have included Black, Latinx, and indigenous communities when the trials were undertaken in the United States.
Why, indeed, do diversity and inclusion matter in these vaccine trials? Dr. Michele Andrasik (Director of Social Behavioral Sciences and Community Engagement and Clinical Professor in the Departments of Global Health and Occupational and Environmental Medicine at the University of Washington) and Dr. Chris Beyrer (Desmond Tutu Professor in Public Health and Human Rights at the Johns Hopkins Bloomberg School of Public Health and professor of Epidemiology, Nursing, and Medicine) have shared in their didactic blog series the following key points:
- Minority communities, particularly Black, Latinx, and indigenous communities, have been particularly hard hit by COVID-19 in the United States as a consequence of high rates of virus transmission, that is, the “force of infection” becomes a major consideration as discussed below.
- These communities also share a legacy of abusive policies and practices, creating social and economic circumstances compromising community health and well-being and fostering mistrust in COVID-19 vaccines; and
- Participation of these communities in COVID-19 vaccine trials is thus of critical importance to ensure vaccine efficacy in the face of intense virus transmission and to build trust in the vaccines.
At one point, the Moderna trial was purposely slowed down to allow for greater recruitment from among these minorities.
“Not because of possible genetic differences,” I underscore, but because the ‘force of infection’ – the epidemiologic risk of exposure to and acquiring the COVID-19 virus –is so much higher for these communities due to their daily life and living circumstances (the housing where they live, the transport they have to take to go to work, the type of jobs they have, the inequalities in health care they receive or lack of them).
Andrasik and Beyrer observe that the early phase of vaccination, upon emergency authorizations of both the Pfizer/BioNTech and the Moderna vaccines, would be marked by “scarcity of vaccine and by a complex set of challenges around allocation, access, and equity.” How those from ethnic and racial minority communities who have experienced such marked health disparities with COVID-19 be included in the allocation framework? What would be the level of their willingness to be immunized if they had not been as engaged during the trials? Their uptake could be markedly lower.
Conceivably, their direct participation in the clinical trials could provide vital information for comparative efficacy of certain specific vaccine types relative to others in light of the greater force of infection in these communities, and not about racial or ethnic differences in biology. Also, their participation would help lessen mistrust of research results with which they have been part of. A feeling of fulfillment engenders public trust in health delivery, especially in public health.
On Vaccine Hesitant, Skeptic and Antivaxxer
Last year, the World Health Organization identified vaccine hesitancy one of the 10 top threats to global health. The problem has remained in some parts of the world. In Russia, even medical doctors hesitate. In Canada, nearly half of our eligible population are eager to receive their dose as soon as available. About 15% are a definitive ‘nay.’ Are these the diehards? While it is truly difficult to fathom why some would remain mistrustful of the 95%-efficacy vaccines without concern of serious harm, we still ask ourselves “How shall we react?”
Respect their viewpoints, I say, and understand the situation. Perhaps, the persons have wounds from self-serving narratives from some of our political leaders. Perhaps, they do not fully comprehend the reasons for the speed, not haste, with which the scientific and medical communities achieved what they did in so short a time than usual – the long preparatory scientific research work done before, the collaborative efforts of research colleagues, the philanthropy from private donors, the partnership with governments, and the innovation on the part of regulatory agencies to evaluating research documents and submissions when applying for interim order or emergency use authorizations for a vaccine product. All these have contributed to accelerated work without compromising the integrity of vaccine development and clinical trials to ensure the safety and efficacy of vaccines.
Tincture of time is needed to heal the wounds. I had taught many youth aspiring to be medical doctors and shared medical advances with fellow physicians. I had attended to patients at intensive care units. I had made house calls. I had seen adults and children as patients. I had done both basic and clinical research. And I even served in appointive and elected public office as a second calling. I have done a lot of community volunteer work. I have come to understand human needs and nature, to some extent.
Accepting the Gift of Science and Medical Progress
I know respect, tolerance, understanding and time are essential to achieving our common goal. I trust all would welcome receiving their COVID-19 jabs – two intramuscular injections to your arm, three weeks and four weeks apart with the Pfizer/BioNTech and the Moderna vaccines, respectively. We do not need a mandate from the outside, but we do need the mandate from inside us. And humanity will be forever grateful. It is pleasing to note, indeed, that the vast majority of Canadians are optimistic of life in 2021 in light of the arrival of the two vaccine products. May we all accept the gift of hard scientific work. Says Dr. Larry Corey, Professor of Medicine and Virology at the University of Washington and Chair of the COVID-19 Vaccine Clinical Trials Working Group: “COVID-19 is a vaccine-preventable disease.”